Research activity
1.Pathophysiology and differential diagnosis
[Research Theme]Pathophysiology and differential diagnosis [On Castleman disease (CD)、TAFRO、IgG-4、POEMS]
[Purpose]
We aim to confirm the histopathological images and clinical findings of CD, TAFRO, IgG-4, and POEMS as well as to gather information on cases reported as being on the boundary and elucidate relationships and differential diagnosis.
[Method]
Through research on case reports, we will investigate into the clinical cases having difficulty in making differential diagnosis, and gather information on the histopathological findings and clinical images. These efforts will lead to elucidation of the disease concepts and diagnostic problems.
[Results and Discussion]
Whether TAFRO syndrome is a subtype of CD or a different disease is the leading problem with regard to differential diagnosis.
The characteristics of the three cases of the first report of TAFRO syndrome are as follows: Fever, systemic edema and breast ascites, hepatosplenomegaly, lymph node swelling, high degree of thrombocytopenia, increased bone marrow megakaryocytes and mild myelofibrosis are observed as the common symptoms. None of these are known single diseases due to nonspecific findings and have not been diagnosed. Both lymphadenopathy and hepatosplenomegaly were mild and poorly characterized as lymphoproliferative diseases. Moreover, in examinations of bone marrow biopsy, liver biopsy (cases 1 and 2), excised spleen (case 2), lymph node biopsy (case 3), neither proliferation of lymphoma cells nor an increase in blood cell phagocytic histiocytes were observed (Takai et al., The Japanese Journal of Clinical Hematology, 51: 320-325, 2010).
In this initial report, a tissue diagnosis had been attempted, but only one case was able to conduct biopsy on a lymph node that was considered to be pathologic, and in the other cases, it should be noted that the lymph nodes were not enlarged enough to be able to be excised. Previous TAFRO research have focused on re-examining the cases diagnosed as CD by re-evaluating the lymph node findings, which have been diagnosed just only based on the tissue findings that appear to be similar to CD like case 3 of the initial report. However, no investigation has been made as to whether both the TAFRO cases with inadequate enlargement of lymph nodes to be recognized as pathological and the TAFRO cases showing tissue finding similar to CD were inclusive within the same spectrum or not.
The diagnostic criteria of TAFRO are mainly made for diagnosis based on clinical findings, and when diagnosing TAFRO using these criteria, there is not much difficulty in distinguishing typical cases including CD. On the other hand, investigation into case reports that describe non-typical cases and boundary cases has identified papers such as "cases of TAFRO syndrome that has difficulties in distinguishing from POEMS syndrome", and "successful treatments with bortezomib and dexamethasone against HIV/HHV-8 negative iMCD with POEMS syndrome", which would be suggestive for considering differential diagnosis. In order to firmly diagnose typical CD, differential diagnosis based on histopathological data of IgG-4 related disease and CD was also considered to be important. We would like to elucidate the diagnostic problems through the research on pathophysiology and differential diagnosis.
[Conclusion]
Establishment of a diagnosis algorithm by examining the position of the TAFRO syndrome cases without lymph node lesion, and cases where pathological conditions are atypical and differential diagnosis is difficult, as well as cases where clinical conditions overlap are the issues that need to be solved.
2.Diagnostic criteria, disease severity classification, revision of clinical practice guidelines and formulation of treatment algorithms
Castleman Disease
Previously, we have published a reference guide for management of Castleman disease including tentative diagnostic criteria and severity classification both in Japanese(Yoshizaki K, et al. Rinsho Ktsueki 58: 97-107, 2017) and in English (Fujimoto S, et al. Mod Rheumatol 28: 161-167, 2018).
We are currently preparing an evidence-based guideline for diagnosis and treatment of Castleman disease in conformity with MINDS (Medical Information Network Distribution Service) promoted by the Japan Council for Quality Health Care (JCQHC), a public interest incorporated foundation. In this guideline, we will put a focus on the formulation of treatment algorithms for idiopathic multicentric Castleman disease (iMCD), which accounts for the majority of Castleman disease in our country and has been registered as one of the designated intractable diseases by the Ministry of Health, Labor and Welfare, Japan.
TAFRO Syndrome
Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome
What is TAFRO syndrome?
It is a new disease concept that Takai et al., proposed in 2010 and stands for thrombocytopenia; anasarca; systemic edema · anasarca (pleural effusion, ascites and edema), fever; reticulin fibrosis in the bone marrow and/or enlargement of megakaryocytes, organomegaly such as hepatosplenomegaly or lymphadenopathy. As lymph node tissues of some of the patients with TAFRO syndrome show histopathological images similar to the mixed type of Castleman disease, some researchers believe that TAFRO syndrome is subtypes of multicentric Castleman disease (Figure 1) .
However, TAFRO syndrome clinically differs from typical multicentric Castleman disease in many ways. For example, many of the multicentric Castleman diseases are relatively chronic, but TAFRO syndrome develops acutely and progresses rapidly. In multicentric Castleman disease, high gamma-globulinemia is observed, platelet counts are increased and enlarged lymph nodes are often found. On the other hand, in TAFRO syndrome, gamma-globulin levels (IgG, IgA, IgM, etc.) are normal and/or rather decreased, platelet counts are decreased, lymph nodes are small, pleural effusion, ascites and edema are prominent, and kidney and/or liver damage are also accompanied. Treatments include high-dose steroid, cyclosporin, tocilizumab, rituximab. Although, in recent years, many patients survive, go into remission and get cured, some patients still develop resistance to treatments (Figure 2).
3.Epidemiological survey, and registry construction and operation
Research on epidemiological clinical practice survey of Castleman disease
Overview
Castleman`s Disease (CD) is a disease in which the lymph nodes become chronically swollen. It is a rare intractable disease whose pathogenesis and pathology are still unknown, and it does not belong to collagen disease or any type of cancer. Because even the disease concept is not yet established, it suffers from low awareness and there are only a few experts. Although the effectiveness of the IL-6 receptor inhibitor (tocilizumab) was shown in 2005, a life-long intravenous administration is required, which hinders daily living and is economically burdensome due to its high cost. Since systemic and epidemiological studies of this disease have not been conducted, the actual condition is not known, and doctors who perform biopsy of lymph nodes for diagnosis are also limited. Since no specific findings other than pathological findings are currently found, it is crucial to discover both diagnostically specific findings and laboratory findings. For this reason, epidemiologic investigation is essential, and this survey is planned to find a resolution for these issues.
Purpose
To grasp the actual current condition of patient care and treatment, establish classification / diagnosis of the disease, disseminate effective treatment regimen and establish of therapeutic guidelines, and aim to prepare clinical guidelines for Castleman Disease.
Background
Castleman disease is a disease advocated by B. Castleman in 1956. After that, it was roughly classified into plasma cell type and hyalin blood vessel type according to the pathological findings of the lymph node. Although the latter can be left unattended for benignancy, the former has various symptoms and laboratory findings and treatment intervention is necessary in many cases, but the concept of the disease has not been established yet. The plasma cell type is further divided into either a disease group positive or negative for HHV-8 virus. In the HHV-8 negative group, the cause is unknown and the condition has not been sufficiently analyzed, and it is called idiopathic multicentric Castleman disease (iMCD), and many of our patients in Japan are iMCD. iMCD is neither a malignant tumor nor a collagen disease but a disease in which the lymph nodes become chronically swollen with persistent inflammation. Although the efficacy of tocilizumab was demonstrated in 2005, it is still poorly controlled, and severe cases could be fatal due to interstitial pneumonia, amyloidosis, advanced anemia merger and other reasons.
If the patient continues to be constantly fatigued and therefore cannot receive appropriate treatment, it gradually progresses to a cachectic state, becoming susceptible to complications and infections, and the patient spends their lifetime in an unsettling condition. The number of doctors who are able to recognize and diagnose CD is extremely limited, and no epidemiologic survey has ever been conducted systematically, so even though the number of patients in Japan is estimated to be 1,500, the actual figure is unknown. One cause of this is that in addition to the fact that diagnostic criteria is solely dependent upon the specific pathological image of the lymph node, there available no serological specific marker and diagnosis cannot be made with other diagnostic methods. Temporary diagnostic criteria and disease activity index were formulated by the group led by Dr. Yoshizaki, which still need to be verified. As mentioned above, CD is a rare disease whose mechanism of pathogenesis is unknown, diagnostic criteria and treatment methods are in the process of being established, and is an intractable disease that requires a long-term treatment. Furthermore, because low awareness of this disease among doctors makes the diagnosis very difficult in addition to the fact that it is a life-threatening disease, a comprehensive epidemiological investigation is required with the cooperation of the patient group. Educational activities for doctors and patients are also necessary. For the reasons described above, Castleman disease is a rare chronic intractable disease that is being left behind, with very little experts and researchers available in addition to its low awareness. This study aims to grasp the actual picture of patient care and treatment, establish disease classification/diagnosis, disseminate effective treatment regimen and establish therapeutic guidelines, and aim for preparation of clinical guidelines for Castleman disease.
4.Central pathology diagnosis
Pathological study aimed at establishing central pathological diagnosis system, improving diagnostic accuracy and elucidation of pathogenesis of Castleman Disease, TAFRO syndrome and its related diseases
[Purpose]
①Castleman Disease, TAFRO syndrome and its related diseases are often difficult to diagnose for non-expert pathologists because of their similarity in histology and rarity. Central pathological diagnosis is implemented with a pathologist board specializing in these diseases so that patients can receive appropriate diagnosis and treatment.
②Pathological investigation about these diseases is performed using residue of pathological specimens in order to elucidate diagnostic pathological factors as well as pathogenesis and pathophysiology of the diseases.
[Method]
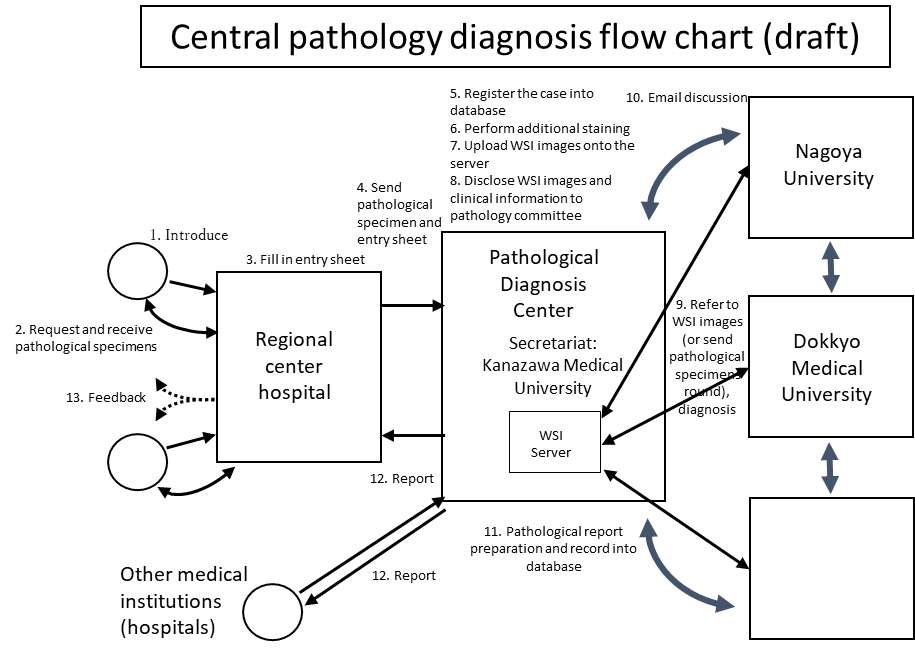
①Central pathological diagnosis
The attending physician at the regional center hospital sends clinical information and pathological specimens to the pathological diagnosis center. Clinical information is registered in the database (see figure). The pathological specimen is subjected to additional staining and histologic images are captured by whole slide imaging (WSI) system and the data is uploaded onto the server. The pathology committee will refer to the WSI images on the server, make a discussion and then make the final diagnosis. Final diagnosis is reported back from the pathological diagnosis center to the doctor of the regional center hospital. Feedback is sent to the hospital where the patient consulted first. Pathological findings are also recorded in the database and are used for clinicopathological study together with the clinical information.
②Pathological investigation using residue of pathological specimens
Pathological investigation such as immunostaining and in situ hybridization is performed on the remaining unstained specimens. Target molecules include surface markers, vascular endothelial markers, and virus-related molecules, which are expected to be of diagnostic significance for these diseases. Cytokine-related molecules, which are expected to elucidate pathophysiology of the diseases, are also investigated.
[Expected Results]
①Central pathological diagnosis
Central diagnosis by expert pathology boards will give more accurate pathological diagnosis. As a result, appropriate treatment will be provided to the patient. In addition, the accumulating information of these rare diseases is expected to elucidate new clinicopathological factors with diagnostic value for these diseases that were previously considered difficult to diagnose.
②Pathological investigation using residue of pathological specimens
Information about expression of various markers and molecules, which are linked to clinical information, will elucidate new diagnostic pathological factors and indicators of therapeutic reactivity. There is a possibility of obtaining new information regarding the pathogenesis and pathophysiology of the diseases as well.
5.Building a hospital network
Mission: Because Castleman disease (CD) is a rare disease, the number of medical institutions that can provide appropriate consultation is limited. Our goal is to construct a medical care system so that any CD patient will be able to receive proper and reliable medical service no matter where they live in Japan.
Plan: The country is divided into 8 areas (Hokkaido, Tohoku, Kanto, Chubu, Kinki, Chugoku, Shikoku, and Kyushu), and medical institutions that will serve as a central core hospital for the medical care of CD will be selected. Regional collaborative institutions, which will partner with the central core hospitals to provide medical service, will also be assembled by referring to the blood disease case registration data from the Japanese Society Hematology, and requests to the candidate institutions will be made by this policy research group (Yoshizaki group). Cooperation between the Yoshizaki group, the central core hospitals and the regional collaborative institutions will create a medical care system available in all 8 areas of Japan.
Accomplishments: 12 institutions - Hokkaido University (Hematology), Tohoku University (Hematology and Rheumatology), Keio University (Hematology), Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital(Collagen disease Department), The Jinkei University (Hematology and Oncology), Kanazawa Medical University (Hematology and Immunology), Japanese Red Cross Nagoya Daiichi Hospital (Hematology), Kyoto University (Hematology and Oncology), Osaka University (Hematology and Oncology), Takamatsu Red Cross Hospital (Hematology), Okayama University (Hematology and Oncology), Nagasaki University (Internal Medicine) were requested to become the regions’ central core hospital (see Figure).
Each central core hospital will 1.) Conduct collaborative clinical research with Yoshizaki group 2.) Work with regional collaborative institutions to promote equal and uniformed medical care for CD 3.) Guide and teach institutions on the medical care of CD 4.) Enforce second opinions, and other important operations.
Next, 236 institutions were nominated as regional collaborative institutions. A survey was taken from these institutions, and 114 had accepted to take on the role as a regional collaborative institution. Furthermore, they were asked to select members that would serve as the primary physician, and 65 institutions (6 from Hokkaido, 3 from Tohoku, 19 from Kanto, 12 from Chubu, 13 from Kinki, 3 from Chugoku, 3 from Shikoku, and 6 from the Kyushu area) have agreed to appoint the attending physician. Therefore, there are currently 12 central core hospitals, and 65 regional collaborative institutions.
Accomplishments up to this point include 1.) Asking 65 institutions to serve as a regional collaborative institution 2.) Creating a mailing list registered via UMIN (University hospital Medical information Network Center) with the Yoshizaki group, the central core hospitals and the regional collaborative institutions 3.) Handing out the “Castleman Disease medical care reference guide” created by the Yoshizaki group to the central core hospitals and the regional collaborative institutions to aim for the improvement in quality for the medical care of CD.
Because both the central core hospitals and the regional collaborative institutions have most of the regions covered, the preparation of the medical care system for CD is near completion.
Future plans: The mailing list will be utilized to share the latest information regarding CD, announce the Yoshizaki group meetings, and distribute minutes of group meetings. In addition, in preparation for medical inquiries from patients, the central core hospitals and the regional collaborative institutions will work together to create a system where anyone will be able to receive proper medical service regardless of where they live.
Current agenda include 1.) Lack of connection between the central core hospitals 2.) Insufficient cooperation system between the central core hospitals and the regional collaborative institutions 3.) Difference in medical standards regarding CD between institutions. Therefore, there is a need to develop a stronger partnership between the central core hospitals and the regional collaborative institutions. We will strive to establish a system where high-quality medical service will be available country-wide through the improvement of CD medical care at both the central core hospitals and the regional collaborative institutions. Requests from patient groups will also be integrated by listening to their opinions.
Figure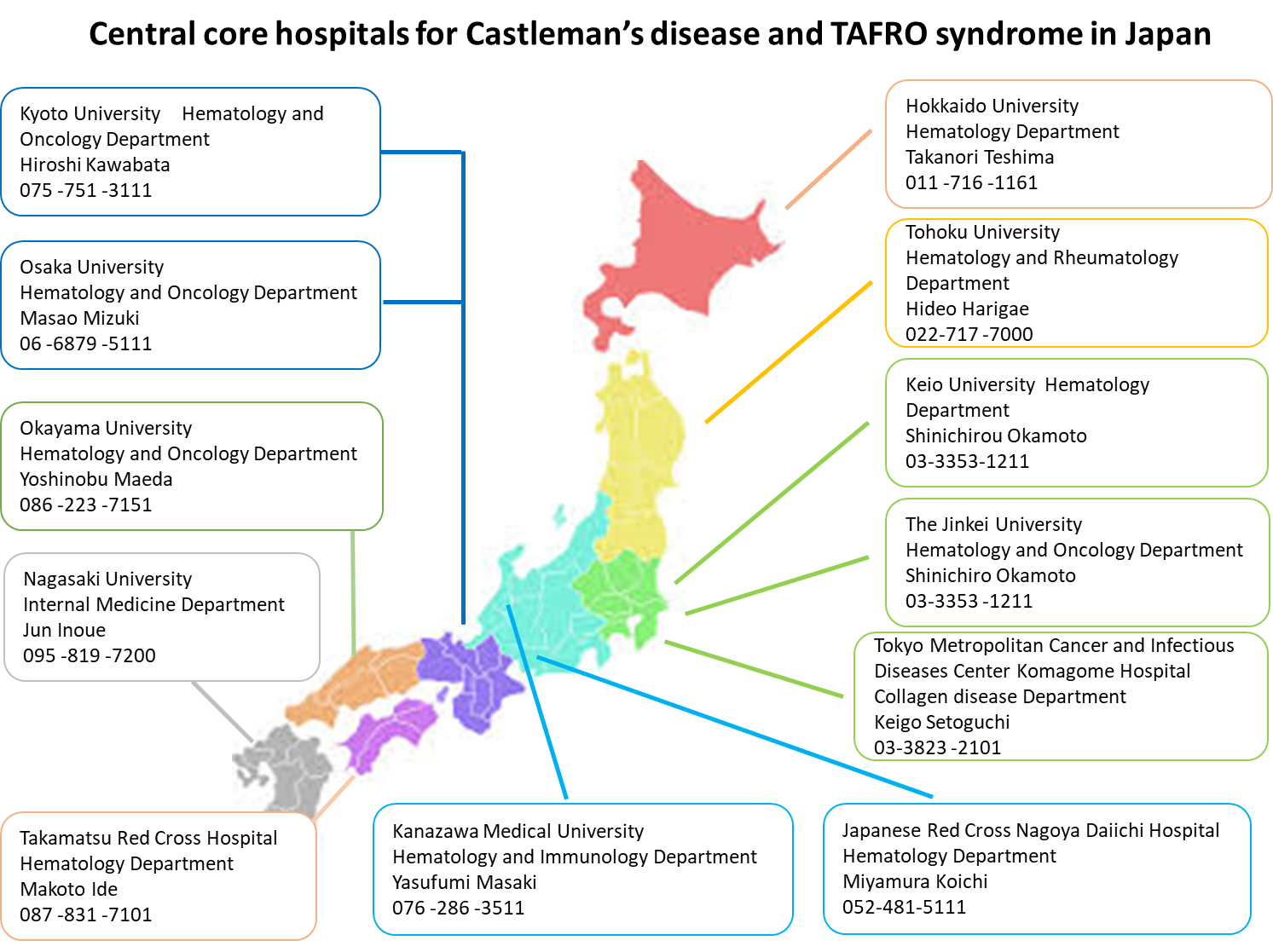
6.International collaboration
Castleman Disease was first described by Benjamin Castleman in 1956, and it is a rare disease that causes chronic symptoms including lymph node enlargement and inflammation. Classifications of this disease include Unicentric Castleman Disease (UCD) where a single region of the lymph node is enlarged, and Multicentric Castleman Disease (MCD), which affects more than one region. The cause is still unknown, but some patients who are HIV or HHV-8 positive have been reported with Castleman disease, which implies some sort of a correlation to the virus infection.
There seems to be a difference with Western countries because while a number of reports on iMCD associated with HIV comes from Western countries, there are few reports on infection-related iMCD from Japan, but there was not enough statistical document, and an international collaborative research was necessary. In regards with treatments, because of its rareness, there were only a small number of case reports from each country, it seems necessary to gather available case reports from each country and analyze them as a whole.
David Fajenbaum, who is a patient of Castleman Disease himself, co-founded the Castleman Disease Collaborative Network (CDCN) in 2012, based in University of Pennsylvania, and started to conduct both basic and clinical collaborative research internationally. CDCN currently runs in 39 countries, with over 420 members. The representative 8 countries (United Kingdom, USA, Brazil, Norway, Japan, France, New Zealand, China) includes 32 members that make up the Scientific Advisory Board (SAB), in which Kazuyuki Yoshizaki, MD, PhD and Makoto Ide MD, PhD of Japan have participated, since the start of CDCN.
The results of an investigation showed that there are certain number of both HHV-8-negative and HIV-negative MCD patients to be present in Western countries, showing that the clinical pictures do not differ significantly from that in Japan. With this, CDCN created an International Diagnostic Criteria for HHV-8-negative/idiopathic MCD, to which both Drs. Yoshizaki and Ide from Japan contributed. An international clinical treatment guideline is currently under development, and by contributing to this efforts, we are trying to include past treatment records and data in Japan to be reflected as much as possible.
Dr. Yoshizaki created an investigation group under the Ministry of Health, Labor, and Welfare in 2015, and started a field survey on patients within Japan. Furthermore, for the first time, a patient group for Castleman disease was founded in the same year, and it aims to raise awareness and activate patient engagement. These actions are expected to make a global expansion in the future.

Worldwide SAB members CDCN websitehttps://www.cdcn.org
7.Patient group support
(1) Castleman Disease
Castleman Disease (CD) is so rare, not even some doctors will be able to recognize this disease, and unfortunately, this leads to patients being not properly diagnosed. The CD research group is working together with the patient group to raise awareness, but we are finding it difficult to even identify the accurate number of patients. We cannot even imagine the anxiety that they are going through.
In 2018, with the help of the patient group, CD was finally recognized as an intractable disease in Japan. Although Tocilizumab (ACTEMRA○R) has been effective in treatment, because the drug does not cure the disease itself but just induce remission, patients need to endure life-long administration. A new medicine from a different perspective is necessary, and this development will not be possible without the help of CD patients. We, with the cooperation of the patient group, will continue to move forward.
We also work as a “bridge” between the research and patient group to answer questions that’s been sent to the patient group. Everything is for the patients.
(2)TAFRO syndrome
The Castleman`s Disease (CD) research group and TAFRO syndrome research group has founded a new research group that includes other related diseases, and patients of TAFRO syndrome has joined the CD patient group and have been working together since. With this, Sadao Aoki MD, PhD (Niigata University of Pharmacy and Applied Life Sciences) and Kazue Takai MD, PhD (Niigata City General Hospital) joined as new advisors of the patient association.
For the 2017 fiscal year, we joined the 5th annual network meeting, and conducted an oral presentation on TAFRO syndrome and its treatment where we communicated with many TAFRO patients. For the 2018 fiscal year, the meeting is planned to take place on September 8th in Niigata City.
In 2018, the hard work of both the research and patient group had paid off, with idiopathic multicentric Castleman disease (iMCD) becoming registered as the 331st intractable disease in Japan. We will continue to research on the pathology and treatment of TAFRO syndrome, as well as support for TAFRO syndrome to be registered as an intractable disease.
8.Research on novel therapies
As a result of the activities of this Policy Research Group of Ministry of Health, Labour and Welfare and the Castleman disease Association in Japan, the Castleman disease has been added to the list of Designated Intractable Disease, and from this year (2018), the burden on seriously ill patients receiving Tocilizumab (TCZ) / Actemra treatment will be ameliorated. Further, the Policy Research Group proposed the use of the TCZ, an anti-IL-6 receptor antibody, as a first line drug from the beginning of treatment for the treatment of idiopathic multicentric Castleman disease (IMCD) . However, for economic reasons, there are many patients who choose untreated follow-up observation or treatment with only steroids. Even if symptomatic improvement is observed by TCZ treatment, there are cases where the therapeutic effect is insufficient that the improvement of the examination value of the inflammatory marker such as CRP can not be sufficiently achieved.
Through the survey of epidemiology, we have found that the treatment effect is insufficient (patients with an abnormal value in any of CRP, Hb, Alb, Ig) in approximately half of patients of each treatment group among no treatment, steroid, TCZ, and steroid and TCZ groups. Apparently, these abnormal values are not completely inhibited by TCZ and suggests the involvement of the activity of a pathway different from the IL-6 signaling pathway in the pathogenic as well as etiologic process of the Castleman disease. As a biologics, TCZ / Actemra, is expensive, and requires an intravenous administration, so that patients are not only economically but also physically suffered, and sucking up time and efforts of medical staff. Therefore, a small molecule therapeutics is much awaited, which will change the situation.
As policy research team, we have been developing clinical guide, severity classification, disease activity index, and disease registry, which can be a good grounding for planning and leading clinical trials of new therapeutic agents for Castleman disease. Through these activities, Dr. Kawabata has proposed the CHAP score, which is a new index to evaluate disease activity with patient QOL taken into account. Meanwhile, in analysis of patient serum samples by Dr. Kawakami and Dr. Uno, blood biomarkers which are considered to be characteristic to Castleman disease and movements before and after the treatment are becoming apparent. Thus, the foundation for preparing a trial protocol for new therapeutic agents for Castleman disease is being established.